A stereo approach to elucidating complex fluids at the nanoscale with Neutrons and X-rays
ILL50-110
ILL50
Understanding the microstructure of complex fluids across multiple scales is essential for building predictive models. While Small Angle Scattering - using neutrons at ILL or X-rays at ESRF - provides invaluable data, fitting individual scattering profiles that have often few features may yields numerical outputs rather than deep physical insights. A paradigm shift occurs when integrating complementary techniques: By combining SAXS, SANS, static and dynamic light scattering with Molecular Dynamics or random Gaussian wavelets simulations, researchers can achieve robust, quantitative analyses—especially when thermodynamic principles, such as those evoked by Maxwell's demon, are incorporated.
This symposium will explore advanced methodologies that bridge scattering techniques with enthalpic and entropic considerations, enabling the development of practical modeling strategies. We will highlight breakthroughs in critical applications, from innovative recycling separation processes to the design of biomimetic colloids with antiviral potential.
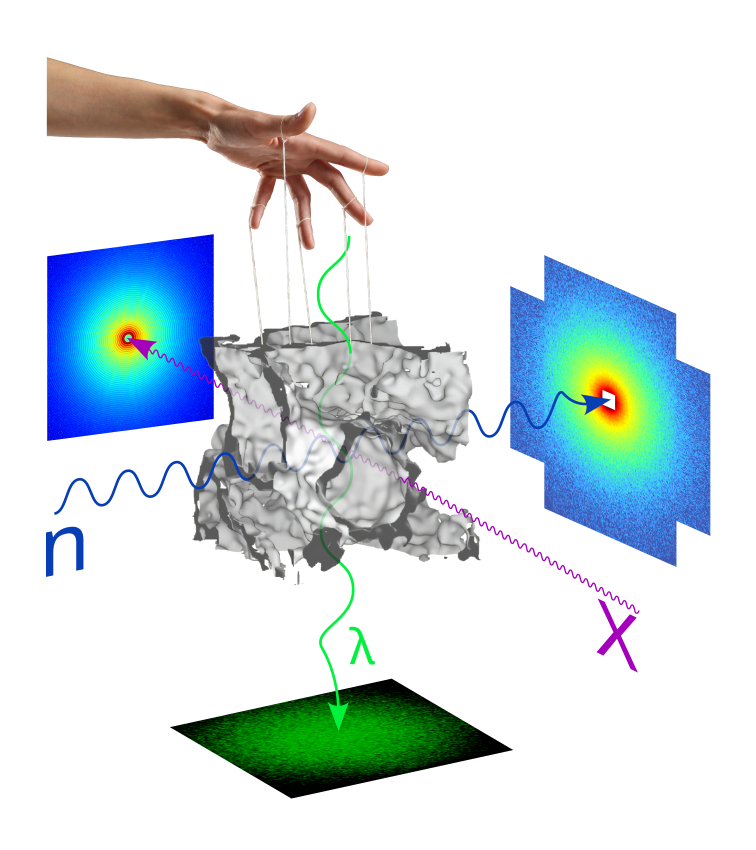
Biliquid foam structure can be observed by neutrons, X-rays and light. Thermal fluctuations controlling the stability are symbolised by the hand of a Maxwell's daemon.
The workshop will also include a special session to honor the career and contributions of Thomas Zemb, a pioneer in the field of colloids and their application in recycling and green processes, as he is moving to an emeritus status 53 years after his first publication with Francis Perrin. In the late 70s, Thomas was building a high sensitivity lab-SAXS as well as a high-resolution USAXS at CEA/Saclay with the help of André Guinier, the father of small-angle X-ray scattering. Later he joined ILL for a year as a technician where he participated in the first experiments on interacting charged surfactant micelles analyzed in absolute scale with both SANS (D11, D17) and SAXS under the supervision of John B Hayter, with Luc Belloni establishing theoretical expressions. In the early 90s, he together with Joan Bordas and Heinrich Stuhrmann advocated for the selection of ID01 and ID02 beamlines at the ESRF. With Peter Lindner, he co-founded the Bombannes summer schools. As the founding director of the Institut de Chimie Séparative de Marcoule (ICSM), he advanced the characterization of colloidal systems through modern colloid science, enhancing separation processes fundamental to hydrometallurgy. His former students and post-docs have used the stereoscopic approach combining X-ray, neutron and light scattering with thermodynamics and simulations. This has led to key progresses in physics, chemistry, biophysics and technology, that will be discussed in this workshop.
-
-
11:30
→
13:30
Lunch
-
11:30
→
13:30
Registration
-
13:30
→
14:00
Welcome with J. Jestin, ILL Science Director
-
14:00
→
15:20
Talks
-
14:00
Heterogeneous decoration of ionic mesopores by ionic and poly(ionic) liquids seen by SAXS and SANS 20m
The molecular structure of mesoporous solid ionic systems is crucial for optimizing macroscopic properties, in particular ionic transport for energy applications. It will be shown how the combination of SAXS and SANS can be used to extract quantitative structural information on the nanoscale by appropriate rescaling in both contrast and scale. We report on the structural analysis of ionic liquid and poly(ionic liquid) embedded in ionosilica matrices, employing a combination of small-angle scattering of neutrons and X-rays, isotopic substitution, and physico-chemical solvent-based extraction methods. Data analysis is based on molecular modelling with an original, quantitative comparison of the scattering curves under different contrasts. In agreement with NMR, it is shown that these mesoporous systems have an unexpected molecular structure, with the ionic liquid counterions penetrating the ionosilica matrix surrounding the mesopores. The poly(ionic liquid) forms patches decorating the pore walls (see Figure 1), with tunable conformation sensitive to solvent conditions.
Speaker: Julian Oberdisse (Université Montpellier) -
14:20
Probing Third Phase Formation in Solvent Extraction: A Joint SAXS/SANS Study 20m
Third-phase formation—an unintended splitting of the organic phase during solvent extraction—creates major operational issues and limits the metal loading that can be safely processed. This demixing arises from the self-assembly of extractant molecules into reverse aggregates whose attractive interactions eventually lead to macroscopic phase separation, but the underlying structural mechanisms remain only partly understood. Using uranium extraction by aliphatic amines (such as tri-octylamine in alkanes) as a model system, we combined SAXS, SANS, and ultra-small-angle scattering to probe the multiscale organization of both the light and third organic phases. The results reveal a hierarchy of structures: small reverse aggregates (4–6 extractant molecules) that cluster into larger uranium-rich domains, water-containing aggregates contributing to high water uptake, and previously unrecognized pockets of trapped diluent dispersed at the nanoscale. Together, the scattering analyses show the coexistence of distinct aggregate populations and dynamic concentration fluctuations, providing new insight into the physical chemistry driving third-phase formation and highlighting the key role of diluent composition and aggregate interactions in phase behavior.
Speaker: Sandrine Dourdain (ICSM) -
14:40
Towards a general understanding of the effects of hydrophobic additives on the viscosity of surfactant gels 20m
Hydrophobic additives, such as essential oils or fragrances, can have a tremendous impact on the viscosity and viscoelasticity of aqueous surfactant gels. The effects are best understood by constructing complete salt curves using sodium laureth sulfate (SLES) in the presence of various additives. A total of four distinct mechanisms of solute-surfactant interactions was identified that differently affect the position and amplitude of the salt curve, resulting in shifts to the left or to the right, or an increase or decrease in viscosity, respectively [1,2]. The effects are intimately linked to the location of the additives within the surfactant film, which is governed by the molecular characteristics of the additives, such as polarity and amphiphilicity.
Mathematical expressions have been established to incorporate each of the four mechanisms into a previously developed analytical model to calculate surfactant salt curves based on differences in packing parameter and chemical potential of three distinct microphases, endcaps, cylinders and junctions [2,3].References :
[1] A. Parker, W. Fieber. Soft Matter 9 (2013), 1203.
[2] W. Fieber, A. Scheklaukov, W. Kunz, M. Pleines, D. Benczédi, T. Zemb. J. Mol. Liq. 329 (2021), 115523.
[3] M. Pleines, W. Kunz, T. Zemb, D. Benczédi, W. Fieber. J. Colloid Interface Sci. 537 (2019), 682.Speaker: Wolfgang Fieber (dsm-firmenich) -
15:00
Understanding Surfactant-Stabilized Oil Foams via X-Ray Scattering 20m
Oil foams can be used in food, cosmetics, and pharmaceutical formulations, for enhancing textures, improving product application, and delivering active ingredients. Traditionally, they have been stabilized with a high concentration of surfactants that form crystalline particles [1]. Recently, oil foams were produced with hydrocarbon-based surfactants, but without the presence of crystalline particles [1]. However, the mechanisms of oil foam formation and stabilization remain underexplored, which is crucial for expanding the relevance of these systems to industrial applications. Here, we used several commercially available surfactants, such as fluid soybean lecithin and formed stable, edible, oil foams, at room temperature. We used a multi-scale approach, to investigate the system from the bulk phase to the air/oil interface by using SAXS/GISAXS and X-ray reflectivity experiments and discovered that the key requirement to promote and stabilize the bubbles was the formation of dense surfactant multilayers, leading to a highly elastic layer [2]. This study offers new insights into oil foam formulation with surfactants already used widely in emulsion formulation, which can facilitate their use in various applications.
References
[1] A.-L. Fameau, and B. P. Binks. Aqueous and oil foams stabilized by surfactant crystals: New concepts and perspectives. Langmuir 2021, 37.15: 4411-4418.
[2] S.-M. Argyri, C. Ugarte Pereyra, R. Bordes, E. Schneck, and A.-L. Fameau. Unravelling the mechanisms of stabilization of edible oil foams using lecithin as a model surfactant, In preparation, 2025.Speaker: Anne-Laure Fameau (INRAE)
-
14:00
-
15:20
→
16:00
Coffee break
-
16:00
→
17:40
Talks
-
16:00
The wonderland of absolute scale and contrast variation in small-angle scattering 20m
A long time ago, Thomas Zemb pointed out that a published study had claimed electron densities corresponding to aluminium in the cores of certain surfactant micelles, something that would have been impossible had absolute-scale modelling been applied together with molecular and concentration constraints. I already appreciated the importance of this approach from my work with contrast-variation SANS at Risø National Laboratory, and I then realized that, to be taken seriously with my SAXS work, I should implement it consistently in my in-house SAXS studies as well. This insight has had a profound impact on my scientific work over the last 25 years.
Thomas Zemb has always demonstrated a deep scientific curiosity, and some of his most characteristic remarks are probably, “This is very interesting (…if it works),” and “They got it totally wrong.” Similar sentiments have influenced my own approach to research: “It should be possible to…” , “It could be fun if…”, and “This cannot be right”.
I will present three projects illustrating this approach to science which I believe align very well with Thomas’ perspective . The first concerns analysis of small-angle scattering from precipitates in Al–Li alloys using a polydisperse hard-sphere model(1). This is an early study showing that the use of absolute scale in both SAXS and SANS (combined with a correct interpretation of the theoretical model) enables determination of precipitate stoichiometry. The second study focuses on contrast-variation SANS of AOT micro-emulsions(2), where we demonstrated how contrast variation can disentangle droplet polydispersity and shape fluctuations. This provided an alternative to neutron spin-echo for assessing shape fluctuations and highlighted the importance of controlling isotope effects in contrast-variation experiments, even when the continuous phase is organic. The third study, more technical in nature, shows that for some systems, combining SAXS with static light scattering and absolute-intensity modelling enables efficient (and inexpensive) in-house contrast variation(3,4). A series of PEP–PEO block-copolymer micelles illustrated the complementarity of light and X-ray scattering, providing detailed structural information and insight into the thermodynamics of the systems.
1 Pedersen, J. S. (1993). Small-angle scattering from precipitates: Analysis by use of a polydisperse hard-sphere model. Physical Review B, 47(2), 657.
2 Arleth, L., & Pedersen, J. S. (2001). Droplet polydispersity and shape fluctuations in AOT [bis (2-ethylhexyl) sulfosuccinate sodium salt] microemulsions studied by contrast variation small-angle neutron scattering. Physical Review E, 63(6), 061406.
3 Jensen, G. V., Shi, Q., Hernansanz, M. J., Oliveira, C. L., Deen, G. R., Almdal, K., & Pedersen, J. S. (2011). Structure of PEP–PEO block copolymer micelles: exploiting the complementarity of small-angle X-ray scattering and static light scattering. Applied Crystallography, 44(3), 473-482.
4 Jensen, G. V., Shi, Q., Deen, G. R., Almdal, K., & Pedersen, J. S. (2012). Structures of PEP–PEO Block Copolymer Micelles: Effects of Changing Solvent and PEO Length and Comparison to a Thermodynamic Model. Macromolecules, 45(1), 430-440.
Speaker: Jan Skov Pedersen (Aarhus University, Denmark) -
16:20
Theory of Liquids applied to colloidal solutions: from the DLVO description to Molecular DFT 20m
The golden age of colloidal physics began in the early 1980s with the simultaneous development of scattering techniques (X-ray, neutron, light) and simple liquid theories (integral equations, Poisson-Boltzmann) applied to the interaction between colloids in solution.
From the very beginning, a very fruitful collaboration with Thomas Zemb led to the writing of numerous practical numerical codes capable of linking, in a few seconds, scattering spectra to microscopic characteristics: size, effective charge, Hamaker constant, depletion, etc.
What was achieved at the time for spherical nanometric particles immersed in a continuous dielectric solvent is now being extended to the level of molecular description, in order to predict the structural and thermodynamic properties of solvation.
Speaker: Luc Belloni (CEA/Saclay) -
16:40
Gravitational Control in Neutron and/or X-ray Scattering via In Situ Centrifugation 20m
In situ centrifugation introduces a controllable gravitational field directly during neutron or X-rays scattering experiments, making it a new thermodynamic control parameter, on par with temperature, pressure, or magnetic fields. By applying high gravitational accelerations (up to several thousand g), it enables real-time observation of structure and phase evolution in soft matter systems (colloidal suspensions, aggregates, micelles, liquid crystals, etc.). We have developed a new generation of sample environment based on a vertical centrifugation principle, using soft centrifugation, meaning between 1 000 g and 6 000 g (g=9.81 m/s2 on Earth). First experiments have been performed on colloidal suspensions of silica nanoparticles (Ludox type) at low volume fraction by neutron scattering (D22, ILL). We have demonstrated how a density gradient is established and stabilized during the centrifugation as function of time at different centrifugal force. While Brownian motion dominates at atmospheric pressure and particles stay dispersed (no sedimentation), under centrifugation the Perrin length drops below 1 µm, a steady state profile is reached in few hours (reversibly) with a very steep gradient at the bottom due to the high Peclet number. All these observables can be tuned to favor aggregation or phase separation, as the short-time kinetics of the gradient formation is known.
Speaker: Christiane Alba-Simionesco (LLB) -
17:00
Microgels as stabilizers for foams: A multiscale approach 20m
Foams appear in many applications such as in personal care products, firefighting and food technology. An elegant tool to tune the foam stability is the addition of polymers of different charge, amphiphilicity or molecular architecture. An example, which will be addressed here are foams which are stabilized by stimuli-responsive microgels.
For understanding macroscopic foam properties, it is important to get deeper insight into the different length scales, i.e. the structuring of microgels at the air/water interface, in foam films, which separate the air bubbles from each other and (macroscopic) foams.
The presentation will focus on microgels based on Poly-N-isopropylacrylamid (PNIPAM). Their stiffness and deformation at the air/liquid interface are controlled by the amount of cross-linker content, which dominates the lateral pattern formation at the liquid interface. A challenge for studies of microgel-stabilized foam films is their massive inhomogeneities, which make it difficult to measure the respective foam film thickness. To get insight into foam film properties, we use a camera-based thin film pressure balance to study microgel-stabilized foam films in terms of disjoining pressure inside the foam films, drainage kinetics, and foam film stability [1, 2]. Film thickness profiles give insights into particle bridging, agglomeration and network formation in the foam films. A correlation is shown with the mechanical properties of the microgels as determined by atomic force microscopy (AFM) nanoindentation measurements. For a complete picture, small angle neutron scattering (SANS) measurements on macroscopic foams provide additional insights into the link between foams and single foam films [1, 3, 4].References
[1] M. Kühnhammer, K. Gräff, E. Loran, O. Soltwedel, O. Löhmann, H. Frielinghaus, R. von Klitzing, Soft Matter, 18, 9249 - 9262, 2022, DOI: 10.1039/D2SM01021F
[2] https://download.hrz.tu-darmstadt.de/media/FB05/SMaI/V0011_Microgel_Foam_Films_KG_v2.mp4
[3] M. Kühnhammer, L. Braun, M. Ludwig, O. Soltwedel, L. Chiappisi, R. von Klitzing, Journal of Applied Crystallography, 55, 758, 2022, DOI: 10.1107/S1600576722004691
[4] M. Kühnhammer, T. Widmann, L. P. Kreuzer, A. J. Schmid, L. Wiehemeier, H. Frielinghaus, S. Jaksch, T. Bögershausen, P. Barron, H. Schneider, A. Hiess, P. Müller-Buschbaum, T. Hellweg, R. von Klitzing*, O. Löhmann. Applied Sciences, 11, 5116-5128, 2021. DOI: 10.3390/app11115116Speaker: Regine von Klitzing (TU Darmstadt) -
17:20
How the formation of ultra-soft microemulsions affects dynamical properties at different lengthscales 20m
This study focuses into the molecular dynamics of the ternary ethanol-octanol-water mixture, selected for its well-defined structure across critical point fluctuations, pre-Ouzo, and Ouzo phases, with organization occurring on a mesoscopic scale of a few nanometers. Using a combination of Neutron Scattering, NMR, rheology, and classical
molecular simulations, we present a detailed description of molecular dynamics across structural domains. A subtle shift in individual dynamics marks the boundary of the monophasic meso-structured region, helping to identify the dynamic signature of the so-called Lifshitz line. Neutron Spin-Echo experiments reveal the collective nature of molecular dynamics, with a de Gennes narrowing effect slowing diffusion in the nano-structured octanol rich region and a dramatic slowdown in droplet diffusion in the pre-Ouzo zone. We eventually discuss a methods for the estimation of the droplets lifetime in such transient organisation, here close to 20 nanoseconds.Speaker: Marie Plazanet (Laboratoire Interdisciplinaire de Physique)
-
16:00
-
17:40
→
18:20
Break: break
-
18:20
→
18:50
Talk by Jean Daillant
-
18:50
→
19:50
Talk by Thomas Zemb
-
19:50
→
21:50
Wine and cheese: Wine and Cheese
-
11:30
→
13:30
-
-
09:00
→
10:00
Talks
-
09:00
SAXS-SANS-based multimodal characterization in batteries 20m
Combining modalities enables revealing the complexity of matter. After having learnt this lesson from Thomas Zemb while studying colloidal systems with him in the years 2000, i later applied it to completely different fields of research – the latter being the operando investigation of battery materials. In this talk i will emphasize the advantages and necessity of SAXS/SANS techniques coupled to other tools as electrochemical methods, neutron/x-ray imaging, or spectroscopic techniques, to visualize and quantify nanoscale changes that happen in electrolyte and electrode materials during device cycling, and their link to the battery performance and longevity. Nanostructured materials as single-ion conducting polymers, nanoengineered silicon-based negative electrodes or hard carbon composites, are key components of emerging technologies for better, safer and more sustainable Li-ion and Na-ion batteries. Synchrotron methods as fast scanning SAXS and SAXS-computed tomography techniques$^1$ are needed to uncover the extended spatiotemporal spaces where multiple reaction and degradation phenomena that happen in a working battery.
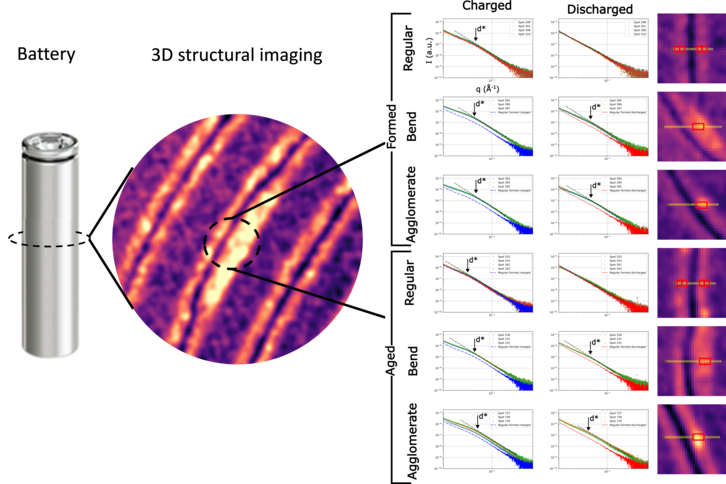
Figure 1. 3D imaging of the interior of a battery using SAXS-CT, enabling to reveal specific defects and areas not lithiating correctly due to active material aggregation$^1$.1: E. Lübke et al, Energy Environ. Sci. 17, 5048-5059 (2024)
Speaker: Sandrine Lyonnard (CEA-IRIG) -
09:20
Multi-scale investigation of the effect of photocurable polyethylene glycol diacrylate on the self-assembly of cellulose nanocrystals (CNCs) 20m
Adding photocurable polymers to aqueous suspensions of cellulose nanocrystals (CNCs) preserves their structural organization after processing, as recently demonstrated for nanocomposites with gradient chiral nematic structure produced by membrane ultrafiltration and UV-curing [1]. Nevertheless,understanding the physiochemical interactions between the nonionic polymer (PEGDA) and the CNCs is necessary to rationalise their structuring under external stimuli. In this work, the effect of increasing amounts of PEGDA (0.7 kDa) on the organisation of CNCs has been investigated with a multi-scale approach, including small angle X-Ray and Light scattering. The results showed a peculiar non-monotonic trend: at low PEGDA/CNC mass ratios (RPC), the polymer adsorbed on the surface of CNCs, blurring their particle morphology and weakening their chiral strength (resulting in a looser cholesteric pitch). Further amounts (RPC=1-1.5) increased the thickness of CNCs, with higher volume fractions and higher chiral strength (smaller pitch). Finally, above RPC=2 (16 wt. %) the amount of PEGDA impacted also the properties of the medium. In fact, a lower dielectric constant increased the electrostatic repulsion between CNCs, while reducing further the pitch period. This complex trend was effectively captured by calculating the twist angle between CNCs, which could be used to describe the behaviour of CNCs suspensions in combination with other additives [2].
[1] Mandin et al., Carb. Pol. 337 (2024), p. 122162.
[2] Metilli et al., J. Colloid Interf. Sci. 658 (2025), p. 476-486Speaker: Lorenzo Metilli (Laboratoire Léon Brillouin) -
09:40
Stochastic models for joint elastic and inelastic scattering data analyses 20m
Models available for scattering data analysis are often specialized to one specific type of data. For examples, a wide array of form- and structure-factors have been developed for analyzing specifically elastic small-angle scattering of x-rays or neutrons (SAXS or SANS). However, when it comes to analyzing inelastic scattering data on the very same systems - say neutron spin-echo (NSE) data - one often has to use different models. Ideally, one would use a single model to jointly analyze all the scattering data available. This would enable one to build on the structural insight from elastic scattering to better understand the dynamical information from inelastic scattering, and vice versa. This presentation illustrates how time-dependent stochastic models can be used for that purpose.
Two classes of models are discussed, namely time-dependent Gaussian-field and Boolean models. They are illustrated on scattering data analysis from micro emulsions (SANS with two contrasts and NSE) 1, from phospholipid membranes subject to bending and thickness fluctuations (SAXS, SANS and NSE) 2, as well as from silica aerogels (SANS and NSE) 3. In all these examples, the data analysis proceeds in two steps: the structural parameters of the model are first inferred from the SAXS or SANS, and the dynamical parameters are identified afterwards from NSE. In other words, inelastic scattering data analysis is used to characterize the time-dependence of the static structures identified through elastic scattering.
1 C.J. Gommes, R. Zorn, S. Jaksch, H. Frielinghaus, O. Holderer. Inelastic neutron scattering analysis with time-dependent Gaussian-field models. J. Chem. Phys. 2021, 155, 024121;
2 C.J. Gommes, P. Dubey, A. Stadler, B. Wu, O. Czakkel, L. Porcar, S. Jacksch, S. Frielinghaus, O. Holderer. Gaussian model of fluctuating membrane and its scattering properties. Phys. Rev. E 2024, 110, 034608;
3 C.J. Gommes. Time-Dependent Hierarchical Model for Elastic and Inelastic Scattering Data Analysis of Aerogels and Similar Soft Materials. Gels 2022, 8, 236.Speaker: Cedric Gommes
-
09:00
-
10:00
→
10:40
Coffee break
-
10:40
→
12:00
Talks
-
10:40
Hierarchical Polymorphism of Semicrystalline Polymers. New Insights Through Combined SAXS/ WAXS/ DSC Results on EVA - Poly(ethylene-co-vinyl acetate). 20m
A new thermo-structural picture of EVA, poly(ethylene-co-vinyl acetate), has been developed by combination of T-scanning SAXS (PDDF analysis), WAXS and DSC results. This expands and supersedes previous, widely accepted ‘lamellar’ models. The basic element is a ‘fringed micelle’ with crystalline PE-rich core and amorphous VA-rich matrix. The chain packing in the core features a temperature-dependent chevron or zig-zag structure, with VA as hinge between PE chains, and a spring-like expansion upon heating that accounts for the strong T-dependent changes in the SAXS long spacing. Before melting to liquid, the PE crystalline chain packing mutates from orthorhombic to hexagonal. Most remarkably, the crystalline core ‘particles’ have similar dimensions in all EVAs of different co-monomer composition studied (9, 16, 28 % VA). At still larger scale, high-resolution SAXS shows that the crystalline domains are embedded in a space-filling 3D cubic lattice of different dimensions, depending on co-monomer composition. This thermo-structural model opens new aspects for, among others, the rational design of EVA-based drug carriers.
Speaker: Peter Laggner -
11:00
Static and Dynamics of Aggregation in Surfactant-Free Ternary Mixtures 20m
Presumably simple solutions can show a variety of nanoscale aggregation structures. Ternary mixtures of three liquids, in which two show only partial mutual solubility, resemble different types of microemulsions even in the absence of classical surfactants. We present fully atomistic molecular dynamics simulations of octanol/ethanol/water mixtures, a typical representative of these “surfactant-free microemulsions”. We compare MD simulations results with different scattering experiments: SAXS/WAXS and neutron scattering reveal the structures present. Neutron Spin-Echo and NMR experiments gives insight into their dynamic behaviour.
References
1) Schöttl, S.; Marcus, J.; Diat, O.; Touraud, D.; Kunz, W.; Zemb, T.; Horinek, D. Emergence of Surfactant-Free Micelles from Ternary Solutions Chem. Sci. 2014, 5, 2949-2954.
2) Schöttl,S.; Lopian, T.; Prévost, S.; Touraud, D.; Grillo, I.; Diat, O.; Zemb, T.; Horinek, D.
Combined MD/SAS analysis of organization on a nanometer-scale in ternary solvent solutions containing a hydrotrope. J. Coll. Inter. Sci. 2019, 540, 623-633.
3) Malayil Kalathil, F. ; Plazanet, M..; Koza, M.M.; Falus, P.; Czakkel, O.; Fouquet, P.; Horinek, D.; Alba-Simionesco, C.; Hoffmann I.
How the formation of ultra-soft microemulsions affects dynamical properties at different length scales J. Mol. Liquids 2025, 432. 127684.Speaker: Dominik Horinek (University of Regensburg) -
11:20
Dynamics of E. Coli in silica nanoparticles 20m
Antimicrobial resistance is rapidly increasing worldwide, calling for alternative strategies beyond conventional antibiotics. Silica nanoparticles (SiO₂ NPs) are promising nanocarriers, but their impact on bacterial dynamics at relevant length and time scales remains insufficiently understood. Here, we combine Ultra Small-Angle X-ray Scattering (USAXS) and X-ray Photon Correlation Spectroscopy (XPCS) at ESRF to investigate the structure and dynamics of Escherichia coli in the presence of Stöber silica nanoparticles of ≈60 nm diameter, either bare (nSiO₂) or covalently functionalized with carbohydrate ligands (Glu1, Glu3, Man1, Gal6). Suspensions of E. coli (OD₆₀₀ = 3.9, 1.17 × 10⁹ cells/mL in phosphate buffer) were mixed with SiO₂ NPs at 0.01 g/L (3.3 × 10¹⁰ NPs/mL). USAXS profiles show no shift in the first minimum, indicating the absence of significant nanoparticle adsorption onto the bacterial surface, while in the intermediate-q range (≈0.06–0.6 nm⁻¹) the bacterial membrane signal is masked by silica scattering. Heterodyne XPCS analysis reveals Brownian-like dynamics (α ≈ 1) for all samples, with relaxation rates scaling linearly with q². Short-time diffusion coefficients remain unchanged within experimental uncertainty upon nanoparticle addition and are independent of the surface functionalization (D₀ ≈ 0.23–0.26 μm²/s), demonstrating that the global bacterial diffusion is not measurably perturbed under these conditions. By quantitatively connecting nanoscale structure and collective bacterial dynamics in situ, this combined USAXS–XPCS framework contributes to a more mechanistic understanding of how nanocarriers behave in biological media, thereby supporting the rational design of more effective nanomedicines.
Speaker: Caroline Silva (Visiting Scientist - ESRF) -
11:40
What have we learnt from SAXS and SANS on nanoparticles made of self-assembled bioconjugates for nanomedicine applications? 20m
In the context of nanomedicine, combining SAXS, SANS and cryoTEM is a powerfull methodology to probe the formation, aging in biological media and interactions of Drug nanoparticles with proteins. In this talk, I will illustrate from different examples of bioconjugates self-assembled nanoparticles, how this approach allowed us to link the nanoparticle’s structure, stability, and analgesic activity [1-6].
- Saha, D., Testard, F., Grillo, I., Zouhiri, F., Desmaele, D., Radulescu, A., ... & Spalla, O. The role of solvent swelling in the self-assembly of squalene based nanomedicines. Soft matter, (2015) 11(21), 4173-4179.
- Rouquette, M., Ser-Le Roux, K., Polrot, M., Bourgaux, C., Michel, J. P., Testard, F., ... & Lepetre-Mouelhi, S.. Towards a clinical application of freeze-dried squalene-based nanomedicines. Journal of drug targeting, (2019) 27(5-6), 699-708.
- Elodie Marret Sicard, phD UPS, « Nanoprécipitation de dérivés squalénés en milieux aqueux : Influence de la formulation sur la distribution de taille et la structure interne des nanoparticules obtenues », Juil. 2019
- Gobeaux, F., Bizeau, J., Samson, F., Marichal, L., Grillo, I., Wien, F., ... & Testard, F. Albumin-driven disassembly of lipidic nanoparticles: the specific case of the squalene-adenosine nanodrug. Nanoscale, (2020) 12(4), 2793-2809.
- Caillaud, M., Gobeaux, F., Hémadi, M., Boutary, S., Guenoun, P., Desmaële, D., ... & Massaad-Massade, L. Supramolecular organization and biological interaction of squalenoyl siRNA nanoparticles. International Journal of Pharmaceutics, (2021) 609, 121117.
- Lepetre-Mouelhi, S., Gobeaux, F., Da Silva, A., Prades, L., Feng, J., Wien, F., ... & Testard, F. Leu-Enkephalin Lipid Prodrug Nanoparticles: Relationship between Nanoparticles’ Structure, Interaction with Bovine Serum Albumin, and Analgesic Activity. Chemistry of Materials, (2024) 36(2), 694-707.
Speaker: Fabienne Testard (CEA, UMR 3685 CEA_CNRS, NIMBE/LIONS Université Paris Saclay)
-
10:40
-
12:00
→
14:00
Lunch
-
14:00
→
15:20
Talks
-
14:00
Combining scattering, molecular simulations, and thermodynamics for the study of surfactant adsorption to fluid interfaces 20m
Adsorption of surfactants to fluid interfaces occurs in technological and daily-life contexts. The surfactant surface coverage $\Gamma$ governs interface characteristics like tension $\gamma$, viscoelastic properties, and the stability of thin foam films. Typical experiments merely yield the tension isotherm $\gamma(c)$, where $c$ is the bulk concentration. Parameter-based models of surfactant adsorption therefore rely on thermodynamic relations between $\gamma(c)$ and the adsorption isotherm $\Gamma(c)$, which are, however, often impractical, so that a direct determination of $\Gamma$ is desirable. We combine various scattering techniques with atomistic molecular dynamics (MD) simulations for the determination of $\Gamma$ for single- and two-component surfactant solutions. Moreover, with the help of free energy calculations in MD simulations we predict $\gamma(c)$ curves for direct comparison and validation with available experimental data. Finally, we combine grazing-incidence X-ray diffraction with MD simulations to characterize structural correlations in surfactant adsorption layers.
Speaker: Emanuel Schneck (TU Darmstadt) -
14:20
Superchaotropic polyoxometalates as smart crosslinkers for cellulose ethers 20m
α-Keggin poyloxometalates (POMs) with low charge density, such as SiW12O404- interact strongly with non-ionic hydrated interfaces driven by a water-mediated driving force, called the chaotropic effect. In aqueous solution of hydroxypropylcellulose (HPC), this binding results in more than 100-fold increase in viscosity at millimolar HSiW-concentrations, and even induces gelation at higher HSiW-concentration at c(HSiW) > 30 mM. By combination of SAXS, SANS, NMR, we show that this thickening effect appears because the POMs bind tightly to the polymer with a binding constant KA= 200 mM-1. The ion-binding leads to electric charging of the polymer at low Siw-concentration, which transitions into physical crosslinking of adjacent polymer chains by the bound ions under screened electrostatics. As Keggin POMs are photocatalysts, photoredox reactions can be employed to reduce the POM, increase its charge density and thus diminish its binding to HPC. We thus demonstrate that photoredox cycles can be used to generate switchable HPC-solutions and gels. The concept of smart chaotropicity for POMs enables a unique and simple strategy to make non-ionic polymers addressable through UV-light for soft material applications.
Speaker: Max Hohenschutz -
14:40
Deciphering the mechanisms of liquid phase separation induced by rabies virus phosphoprotein 20m
Rabies virus (RABV) generates membrane-less liquid organelles (Negri bodies) in the cytoplasm of its host cell, where genome transcription and replication and nucleocapsid assembly take place, but the mechanisms of their assembly and maturation remain to be explained. An essential component of the viral RNA synthesizing machine, the phosphoprotein (P), acts as a scaffold protein for the assembly of these condensates. This intrinsically disordered protein forms star-shaped dimers with N-terminal negatively charged flexible arms and C-terminal globular domains exhibiting a large dipole moment. Our study shows that in vitro self-association of RABV P drives a complex thermoresponsive phase separation with a lower critical solution temperature. Protein dimers assemble already below the saturation concentration, and condensation is driven by attractive conformation-specific interactions leading to reentrant liquid phase separation over a narrow range of salt concentration. We propose a minimal molecular model in which P can adopt three limit conformational states and the disordered N-terminal arms control the interactions between giant dipoles that is consistent with our observations.
Speaker: Marc Jamin (Université Grenoble Alpes) -
15:00
From synthesis to self-propulsion: Bimetallic Janus nanocrystals 20m
Bimetallic Janus nanocrystals result from integrating two distinct metals side-by-side into one single particle$^1$. This asymmetric arrangement allows the unique properties associated with each metal to be incorporated in tandem. When dispersed in an appropriate catalytic medium, such particles exhibit self-propulsion mediated by diffusiophoresis$^{2,3}$. Before these properties can be exploited, robust and scalable synthesis protocols are required. Here we demonstrate the preparation of bimetallic palladium-silver Janus nanocrystals using a seed-mediated growth method$^4$. The kinetically controlled overgrowth of plasmonic Ag caps on the Pd seeds is followed by coupling in-situ small-angle X-ray scattering (SAXS) with UV-Vis absorption spectroscopy. Currently these self-propelled systems are typically studied at low volume fractions. Moving forward with a robust synthesis and sufficient yield of these particles, it will be possible to realize self-propelled or active colloids at high volume fractions. SAXS and X-ray photon correlation spectroscopy (XPCS) will be used to probe the interactions and dynamics in these active systems. Furthermore, the asymmetry of particles enables to access the rotational diffusion by XPCS. This work provides a groundwork for future studies into the diffusiophoretic self-propulsion of active colloids, which mimic certain collective behavior of living systems.
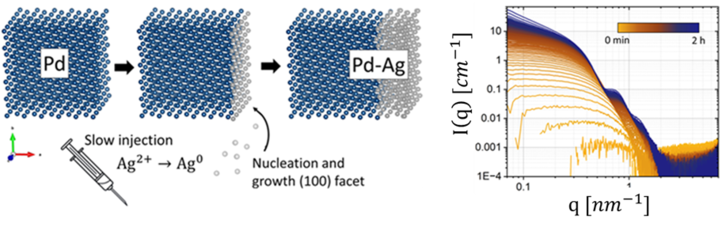
Figure 1. Schematic illustration of Pd-Ag growth through controlled injection rate (left). In-situ SAXS patterns of palladium seed growth (right).References
1: Qiu J. et al. Bimetallic Janus Nanocrystals: Syntheses and Applications. Adv. Mater. 2022, 34, 2102591.
2: Zinn T. et al. Emergent dynamics of light-induced active colloids probed by XPCS. New J. Phys. 2022, 24, 093007.
3: Zhu W. et al. Exploring the Synthesis of Self-Organization and Active Motion. J. Phys. Chem. Lett. 2024, 15 (20), 5476-5487.
4: Zeng, J. et al. Controlling the Nucleation and Growth of Silver on Palladium Nanocubes by Manipulating the Reaction Kinetics. Angew. Chem. Int. Ed. 2012. 51: 2354-2358.Speaker: Austin Hubley (ESRF)
-
14:00
-
15:20
→
16:00
Coffee break
-
16:00
→
17:40
Talks
-
16:00
Survival of the FITtest: Can Evolution Teach Us to Fit SAS Data? 20m
Small-angle scattering (SAS) curve fitting is frequently posed as a rugged, high-dimensional optimisation problem. In many practical cases, closely similar I(q) profiles can be produced by markedly different parameter combinations, highlighting the challenge of parameter degeneracies. Satisfactory solutions are rarely achieved without imposing constraints or introducing prior assumptions about the sample, acknowledging the complexity researchers face.
Genetic algorithms (GAs) have long been used in SAS data analysis. Indeed, GA usage can drastically simplify the analysis of experiments. However, standard single-population implementations (often bit-encoded) perform poorly as dimensionality and complexity increase or when the forward model involves strongly coupled continuous parameters. Here, it will be shown how strategies inspired by Darwinian Evolution (variation, selective pressure, adaptive mutation, spatial separation, and limited exchange) can be translated into computational optimisation schemes for SAS data. In particular, an island-model evolutionary algorithm is presented, in which multiple populations are evolved in parallel under distinct evolutionary pressures and represent different real-space modelling hypotheses. The Galápagos finches, a well-known example of adaptive radiation, inspire such a GA design. These design principles are then consolidated in STORM (Small-Angle Scattering Toolkit for Optimisation and Real-space Modelling), which implements a low-prior, user-friendly, evolution-inspired philosophy designed to provide robust SAS fitting in highly dimensional settings.
Speaker: Rodrigo Sanches Pires (European Synchotron Radiation Facility, ID02 beamline) -
16:20
Membrane diffraction under controlled humidity: a tool to probe phase diagrams, distances and molecular forces in synthetic and natural lamellar systems 20m
There are many examples of synthetic and biomimetic lamellar organisations in surfactant and lipid systems, but there are only a few examples of such membrane multilayers in nature: the myelin sheet in vertebrates, stacked thylakoid membranes in photosynthetic organisms and the lamellar domains of the stratum corneum. Whether synthetic or natural, these examples have in common highly ordered lamellar structures or domains, an organization that requires a subtil balance between attractive and repulsive interactions, and between stiffness and flexibility to prevent collapse or unbinding.
As Thomas taught me, whether lamellar or non-lamellar, these structures can be probed by small-angle scattering techniques on bulk samples under controlled osmotic pressure and/or using samples oriented on a solid substrate by membrane diffraction under controlled relative humidity. In both techniques the osmotic pressure of the sample is controlled along dilution lines to investigate the phase diagram, the structure of the phases (distances, order) and the forces at play by determining the pressure-distance curves. By means of polymer solutions or under controlled relative humidity these forces can be identified and quantified analytically or using MD simulation at known osmotic pressure or water chemical potential.
In this short talk, I will present 3 examples of increasing complexity: a charged synthetic lipid system (DOPS), a complex quaternary lipid model of thylakoid membranes, and myelin from the central and peripheral nervous systems of mouse and rat nerves.
Speaker: Bruno Demé (Institut Laue-Langevin) -
16:40
Operando X-ray scattering and imaging experiments to reveal the multiscale-structure of bone scaffolds made of nanoparticles for regenerative biomaterials. 20m
A new-generation of synthetic bone scaffold is tailored using a bricks-and-mortar approach from bioactive glass nanoparticles BGNps and customized polymers (PLA, poly (lactic acid), the mortar). Used as synthetic implants for substitutive and regenerative therapies targeting mandibular osteoradionecrosis (ORM). To shed light on the mechanisms behind the formation of the hierarchical structure of these scaffolds, the synthesis of BGNps was studied using in-situ SAXS at synchrotrons and thanks to a custom-built sample chamber, fast X-ray phase-contrast tomography operando experiments.
Speaker: Guillaume Brotons (Université du Mans) -
17:00
Towards a renaissance of quantitative light scattering method of Gouy beyond the Tyndall effect ? 20m
Louis-George Gouy (1853–1926) discovered in the late 19th century that static light scattering exhibits intense scattering at 90 degrees. This phenomenon was related to back-scattering, and he was able to quantify it by comparing its intensity to the intensity of diffraction produced by the edge of a screen—the latter being the subject of most interest at the time.
Moreover, Gouy related the angle of maximum scattering to the concentration of the medium. This key finding later allowed researchers like Rayleigh, Smoluchowski, and Perrin to quantitatively determine the concentration of colloids per unit volume.
Modern Application and TechnologyModern technology, such as the Vasco-Kin device developed by Cordouan Technology, has significantly advanced this field. By upgrading the instrument and incorporating a careful determination of the absolute value of the Rayleigh ratio—in combination with advanced time-resolved filtering—it is now possible to determine not only the apparent size but also the concentration and Rayleigh ratio of colloidal solutions and complex fluids. This is achievable even in the presence of "dusts" that typically produce intense, dominant, and parasitic scattering signals.
In the q-range limited by the scattering geometry, we utilize a massive photon flux of 5×10$^{15}$ photons/s. This flux is approximately 100 times greater than the intensity available at the world's best beamline (ID02) operating at 12 keV.

Louis-George Gouy (1853–1926) is shown in front of his original light scattering instrument (source: Wikipedia). This historical apparatus can be compared to its modern implementation, the portable Vasco-Kin device by Cordouan Technology. The Vasco-Kin counts up to 10$^{6}$ photons per second, offering an effective dynamic range of four decades for the scattered light induced by a laser beam with a flux exceeding 10$^{15}$ photons/s. This allows for studies on the probability of diffusion by colloids, even after filtering from dust, with sensitivity as low as 10$^{−10}$.Speaker: Thomas Zemb (CEA/ICSM) -
17:20
Farewell 20m
-
16:00
-
09:00
→
10:00
